Dosing information for treatment with TRODELVY
TRODELVY dosing schedule

TRODELVY dose of 10 mg/kg is given as an intravenous (IV) infusion

Doses are given once a week for 2 weeks (Day 1 and Day 8) of 21-day treatment cycles

Each treatment cycle is 21 days (3 weeks)
Sample 21-day treatment cycle
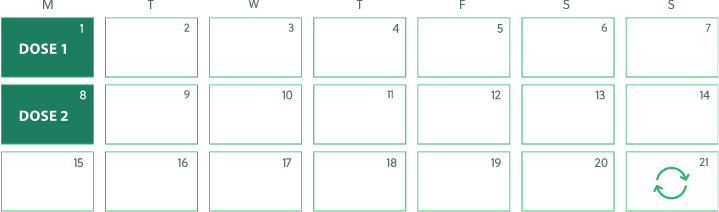
Treatment cycles repeat every 21 days
You and your healthcare provider will decide how many treatment cycles you receive. This may be based on factors such as whether your tumor has responded to treatment or your body’s ability to tolerate treatment.
What to expect on treatment days
To prepare for each treatment, your healthcare team may:
- Measure your weight to find the right dose
- Perform a short physical exam to check your blood pressure, pulse, breathing, and temperature
- Put an IV tube in your arm, unless you already have a port which can be used for the infusion
- Take a blood sample
Before starting TRODELVY, tell your healthcare provider about any medicines you are taking. Be sure to include prescription and over-the-counter medicines, vitamins, and herbal supplements. Certain medicines may affect the way TRODELVY works.
Once it’s time for treatment, you can expect a 3-step process:
After each infusion, your healthcare team will watch you for reactions for at least 30 minutes. If you experience any side effects while taking TRODELVY, tell your healthcare provider right away. Please read the Important Safety Information below and the information on side effects.
Next steps after treatment is complete
Your healthcare provider may give you medicines to take home that can help you manage possible side effects of TRODELVY. Keep track of when and how often side effects occur, as well as their severity, so your healthcare provider can best support you. Call your healthcare provider right away if you experience any side effects. Please review these Important Facts for more information.
Be sure to tell your healthcare team about any side effects you have while on TRODELVY.
TRODELVY® (sacituzumab govitecan-hziy) is a prescription medicine used to treat adults with triple-negative breast cancer (negative for estrogen and progesterone hormone receptors and HER2) that has spread to other parts of the body (metastatic) or cannot be removed by surgery, and who have received two or more prior treatments, including at least one treatment for metastatic disease.
It is not known if TRODELVY is safe and effective in people with moderate or severe liver problems or in children.
Important Safety Information
TRODELVY can cause serious side effects, including low white blood cell count and diarrhea:
Tap for Important Safety Information. TRODELVY can cause serious side effects, including low white blood cell count and diarrhea:
- Low white blood cell count (neutropenia) which is common and can sometimes be severe and lead to infections that can be life-threatening or cause death. Your healthcare provider should check your blood cell counts during treatment. If your white blood cell count is too low, your healthcare provider may need to lower your dose, give you a medicine to help prevent low blood cell count with future doses of TRODELVY, or in some cases may stop TRODELVY. Your healthcare provider may need to give you antibiotic medicines if you develop fever while your white blood cell count is low. Call your healthcare provider right away if you develop any of the following signs of infection: fever, chills, cough, shortness of breath, or burning or pain when you urinate.
- Severe diarrhea. Diarrhea is common and can be severe. Severe diarrhea can lead to loss of too much body fluid (dehydration) and kidney problems. Your healthcare provider should monitor you for diarrhea and give you medicine as needed to help control it. If you lose too much body fluid, your healthcare provider may need to give you fluids and electrolytes to replace body salts. If you develop diarrhea during your treatment with TRODELVY, your healthcare provider should check to see if it may be caused by an infection. Your healthcare provider may decrease your dose or stop TRODELVY if your diarrhea is severe and cannot be controlled with anti-diarrheal medicines.
- Call your healthcare provider right away the first time that you get diarrhea during treatment with TRODELVY; if you have black or bloody stools; if you have symptoms of dehydration, such as lightheadedness, dizziness, or faintness; if you are unable to take fluids by mouth due to nausea or vomiting; or if you are not able to get your diarrhea under control within 24 hours.
Do not receive TRODELVY if you have had a severe allergic reaction to TRODELVY. Ask your healthcare provider if you are not sure.
Allergic and infusion-related reactions which can be serious and life-threatening. Tell your healthcare provider or nurse right away if you get any of the following symptoms during your infusion of TRODELVY or within 24 hours after: swelling of your face, lips, tongue, or throat; hives; skin rash, itching, or flushing of your skin; fever; difficulty breathing or wheezing; lightheadedness, dizziness, feeling faint, or pass out; or chills or shaking chills (rigors).
Nausea and vomiting are common with TRODELVY and can sometimes be severe. Before each dose of TRODELVY, you will receive medicines to help prevent nausea and vomiting along with medicines to take home with instructions about how to take them. Call your healthcare provider right away if you have nausea or vomiting that is not controlled with the medicines prescribed for you. Your healthcare provider may decide to decrease your dose or stop TRODELVY if your nausea and vomiting is severe and cannot be controlled with anti-nausea medicines.
Before receiving TRODELVY, tell your healthcare provider about all of your medical conditions, including if you:
- have been told that you carry a gene for UGT1A1*28, which can increase your risk of getting side effects with TRODELVY, especially low white blood cell counts, with or without a fever, and low red blood cell counts.
- have liver problems.
- are pregnant or plan to become pregnant. TRODELVY can harm your unborn baby. Your healthcare provider should check to see if you are pregnant before you start receiving TRODELVY. TRODELVY may cause fertility problems in females, which could affect your ability to have a baby. Talk to your healthcare provider if fertility is a concern for you.
- Females who can become pregnant should use effective birth control during treatment and for 6 months after your last dose of TRODELVY. Talk to your healthcare provider about birth control choices that may be right for you during this time. Tell your healthcare provider right away if you become pregnant during treatment with TRODELVY.
- Males with a female partner who can become pregnant should use effective birth control during treatment and for 3 months after your last dose of TRODELVY.
- are breastfeeding or plan to breastfeed. It is not known if TRODELVY passes into your breastmilk and can harm your baby. Do not breastfeed during treatment and for 1 month after your last dose of TRODELVY.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Certain medicines may affect the way TRODELVY works.
The most common side effects of TRODELVY include decreased white blood cell (leukocyte and lymphocyte) and red blood cell counts, feeling tired or weak, hair loss, constipation, increased sugar levels in the blood, decreased protein levels (albumin) in the blood, decreased appetite, changes in kidney function test, increased levels of enzyme called alkaline phosphatase in the blood (test for liver or bone problems), and decreased levels of magnesium, potassium, and sodium in the blood.
These are not all of the possible side effects of TRODELVY. Call your doctor for medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Please click to see Important Facts about TRODELVY, including Important Warning.




